SAS accelerates delivery of novel medicines using AI and analytics
Cooperation with AstraZeneca for more innovation in clinical research
Advertisement
SAS, a leader in AI and analytics, is helping to revolutionize the use of clinical trial data so new medicines can be delivered to patients faster than before. After a thorough evaluation, SAS has been chosen by global biopharmaceutical company AstraZeneca to help increase efficiency and drive automation in the delivery of statistical analyses for clinical and post-approval submissions to regulatory authorities, via SAS’s cloud-based software and technologies.
SAS will support the redesign of clinical and patient data flow by delivering industry-leading analytics and AI, manage changing trial designs in a fast-evolving regulatory environment, enable data re-use, and help accelerate reporting and submission timelines. It will also deliver increased capacity, automation, interoperability, and flexibility to bring in and analyze diverse and novel patient data sources – such as those coming from wearables, sensors and precision medicine – as part of the submissions process.
This will be achieved by supporting the analysis and reporting phases with SAS® Life Science Analytics Framework and SAS® Viya®, a scalable and powerful cloud-based industry platform enabling swift decision-making regardless of data volumes or complexity using modern cloud technologies. This has the potential to provide significant productivity gains by driving faster time to market and reduced IT costs. The SAS and AstraZeneca partnership will enable teams across the organization to collaborate and increase clinical research innovation.
Christopher J Miller, VP Biometrics at AstraZeneca, said, “This partnership with SAS supports the transformation of how we use clinical data to support our patient-centric approach and focus on getting medicines to patients faster than ever before. It will also allow us to introduce new ways of working and embrace new technologies and trial models to accelerate our portfolio.”
Bryan Harris, SAS Executive Vice President and Chief Technology Officer, said, “I’m delighted that SAS is building on the strong relationship it has had with AstraZeneca over many years by being part of this transformation program. The work they do positively impacts the lives of millions of people around the world.
“This is exciting because we have solidified a great foundation between our companies, but we also recognize we are just scratching the surface. We pay attention to technology and the advancements in AI, and we thrive on thinking through how our technology blended with AstraZeneca’s expertise and insight can create new medical solutions for their customers.”
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Stockholm-based biotech start-up aims to develop the next generation of cell therapies - Neogap Therapeutics' PIOR technology identifies targets for personalised liver cancer immunotherapies

Millions in funding for the development of a novel drug against multi-resistant bacteria - Infex and Justus-Liebig-University Giessen awarded £1 million grant from PACE
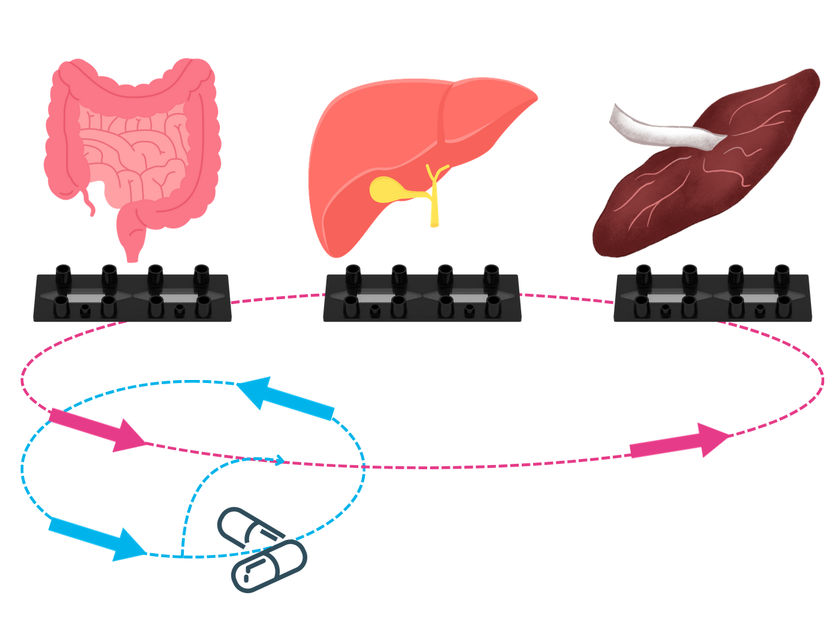
“Multi Organ Model” - Animal-free alternative for pharmacokinetics research - Dynamic42 and research partners present three-organ system to reduce animal testing

New methods for reliable evaluation of chemicals and pharmaceuticals - 18 partners from eight countries are working on the introduction of innovative methods for evaluating safety and efficacy without animal testing

New approaches in cancer research - Tumour-on-chip model supports preclinical drug development on pancreatic cancer

The EPO unveils the top 10 innovators of the first standalone edition of the Young Inventors Prize 2025 - Prize recognises innovators under 30 who are tackling Global Challenges through groundbreaking science and technology

NUCLIDIUM Closes EUR 84 Million Series B Financing - "NUCLIDIUM’s platform stands out in a rapidly evolving field and will change how radiotheranostic care is delivered"

Life Science Pitch Day: Where Visionaries Meet Investors - Start-ups presented their innovative approaches to solving urgent medical challenges

AmphiStar secures €12.5m EIC funding to bring its biosurfactants to market - Belgian start-up impresses with sustainable microbial biosurfactants, upcycled from a bio-based waste feedstock

Double Excellence: ibidi Recognized for Outstanding Business Success and Family Friendliness

BIOTECH AUSTRIA calls for better framework conditions for the biotechnology sector of the future - Proposals provide impetus for research, infrastructure and investments in Austria