New biotech venture PHIOGEN to tackle the global threat of antimicrobial resistance
By optimizing nature’s defenders, the team has produced unprecedented phage treatments
Advertisement
The new biotech venture PHIOGEN is a spin-off company from Baylor College of Medicine’s TAILOR Labs, one of the United States only academic phage therapy cores with a decade’s worth of revolutionary research related to bacteriophages, viruses that infect and destroy bacteria.
The company made its debut at the 6th World Conference on Targeting Phage Therapy in Paris, June 1-2, 2023.
PHIOGEN’s R&D efforts are led by phage researcher Dr. Anthony Maresso, founder of TAILOR Labs and associate professor of molecular virology and microbiology at Baylor, whose phage therapy work has attracted funding of more than $5 million to date.
The globally research team behind PHIOGEN is housed in the world’s largest medical complex inside the prestigious Texas Medical Center’s Innovation Hub.
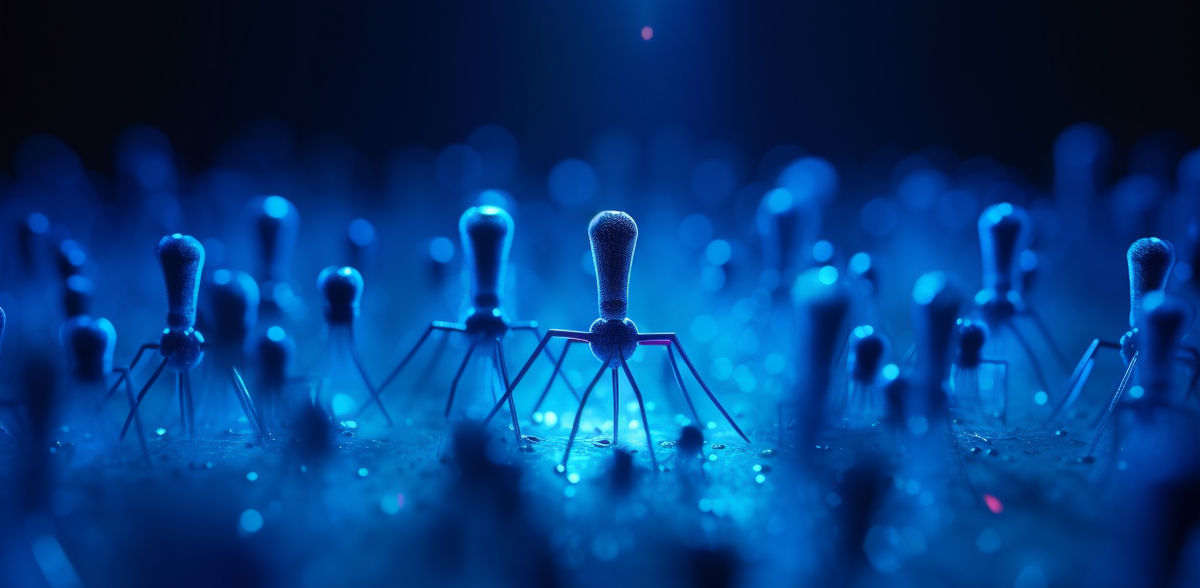
PHIOGEN has developed a world-first technology platform that mobilizes the natural power of bacteriophages to tackle critical and life-threatening infections. This marks a significant medical breakthrough for countering the global threat of antimicrobial resistance.
The World Health Organization deems drug resistant infections as one of the top 10 global public health threats facing humanity with estimates of over 5 million deaths worldwide attributed to antibiotic resistant infections.
The proprietary first-of-its-kind technology platform that is being spearheaded by PHIOGEN is able to discover and screen at-scale naturally occurring bacteriophages, singling out those with elite bacteria-fighting abilities, and directing biological changes to evolve the phage into antimicrobials that overcome resistance.
This creates a new business model for phage therapy as the group is able to create products that treat populations of people instead of on a per patient basis. By optimizing nature’s defenders, the team has produced unprecedented phage treatments which have already successfully saved the lives of several patients in FDA approved, compassionate use cases.
“We receive high-performing phage fighters that are trained and ready to deliver safe and effective treatments for clinical applications,” said Amanda Burkardt, CEO at PHIOGEN.
Other news from the department business & finance
Most read news
More news from our other portals
Something is happening in the life science industry ...
This is what true pioneering spirit looks like: Plenty of innovative start-ups are bringing fresh ideas, lifeblood and entrepreneurial spirit to change tomorrow's world for the better. Immerse yourself in the world of these young companies and take the opportunity to get in touch with the founders.