Leadership change at Köttermann B.V. in the Netherlands
Advertisement
As of 30 June 2025, the Köttermann Group will see a leadership change at its Dutch subsidiary, Köttermann B.V.: Roeland Wegman will step down from his position as Managing Director. The Köttermann Group thanks Roeland Wegman for his dedication and contribution to the management of the Breda site. “We wish him all the best for the future, both personally and professionally,” says Kazim Doyuran, Managing Director of Köttermann GmbH and the Köttermann Group.
Kenneth de Paepe has been with the Köttermann Group for many years and has successfully led the Belgian subsidiary, Köttermann bvba - also responsible for Luxembourg - since 2018. A seasoned industry expert, he brings extensive market knowledge and strategic insight to the role. “I am very much looking forward to this new role in the Netherlands and to strategically expanding our activities across the Benelux region together with the local team,” says Kenneth de Paepe. “Köttermann stands for quality, safety and tailored solutions, values I am committed to further strengthening across all three countries.”
“The Benelux region is a key growth market for us. With Kenneth de Paepe, we have an experienced leader who will consistently pursue our strategic goals, business development strategy and actively connect our sites across all three countries,” adds Kazim Doyuran.“Kenneth has a proven track record in Belgium, having successfully grown the business while continuously striving to deliver better solutions in the field of
laboratory infrastructure.”
Most read news
Other news from the department people

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

1.2 million US dollar award honors pioneering work in cryo-electron tomography - Wolfgang Baumeister receives prestigious Shaw Prize

Leon-nanodrugs appoints Dr. Wolfgang Hofmann as Chief Executive Officer

Refeyn Announces Leadership Transition - Jonathan Flint Steps Down as Chairman, Jean-Paul Mangeolle Appointed as Successor

Joachim Kreuzburg, Former CEO of Sartorius, Joins Cube Biotech’s Board of Directors
New high-tech startup developing smart contact lenses for glaucoma diagnosis and management
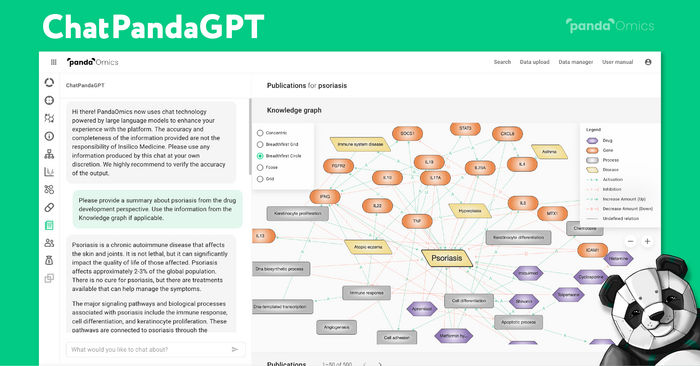
Insilico Medicine brings AI-powered “ChatPandaGPT” to its target discovery platform - A revolution in drug discovery thanks to a new chat assistant?
Insilico Medicine-led study combines quantum computing and generative AI for drug discovery - “Quantum computing is recognized as the next technology breakthrough which will make a great impact, and the pharmaceutical industry is believed to be among the first wave of industries benefiting from the advancement”

Waters Corporation and Princeton University Announce Multi-Year Research Collaboration - Agreement to take on real-world challenges in biologics and materials research

New biotech venture PHIOGEN to tackle the global threat of antimicrobial resistance - By optimizing nature’s defenders, the team has produced unprecedented phage treatments
CureVac and MD Anderson enter strategic collaboration to develop novel cancer vaccines - Collaboration aims to develop novel, off-the-shelf, mRNA-based cancer vaccines in selected hematological and solid cancers with high unmet medical need
CureVac Advances Cancer Vaccine Candidate CVGBM to Part B of Phase 1 Study in Patients with Resected Glioblastoma - First patient administered in dose-confirmation Part B of Phase 1 study with mRNA-based, multiepitope cancer vaccine candidate CVGBM