CureVac enters clinical phase with mRNA vaccine
First data expected on intramuscular application of the newly developed vaccine
Advertisement
CureVac AG, a fully integrated biopharmaceutical company pioneering the field of mRNA-based drugs, today announced the initiation of its Phase I dose-escalation clinical trial with the novel mRNA-based rabies vaccine, CV7202. This is the first-in-human clinical trial of CureVac’s naturally optimized mRNA technology delivered using a lipid nanoparticle (LNP), tailored to provide the vaccine with a strong, safe and persistent immune response. The study will assess the safety, reactogenicity, and the potential protective immune response of the vaccine.
CV7202 is a prophylactic mRNA-based vaccine encoding the rabies virus glycoprotein, RABV-G, formulated with next generation LNP. CureVac’s platform of mRNA-based therapeutics optimizes the properties of mRNA, including stability and immunogenicity, using enhanced sequences of naturally occurring building blocks. CureVac’s technology stimulates the immune system to mount a response against an antigen of choice, potentially providing potent prophylactic vaccines for the prevention of infectious diseases, such as rabies, as well as immunotherapies for the treatment of cancer.
“The first study participant enrolled in this rabies clinical trial is a significant milestone for CureVac, and allows the company to demonstrate its ability to trigger an immune response in vaccine naïve populations, which is different from vaccines just boosting an already existing immune response such as a flu vaccination,” said Dan Menichella, Chief Executive Officer of CureVac. “Our goal is to significantly improve today’s commercial three-to-five shot regimen rabies vaccines with a one or two shot solution that has a markedly longer duration. With this trial, we are taking an important step toward further validating the potential of our mRNA-based vaccine platform and therapeutic pipeline that will soon include additional oncology and rare diseases candidates.”
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
New drug candidates reverse drug resistance in multiple myeloma in preclinical models
Elevated hormone flags liver problems in mice with methylmalonic acidemia - Study findings can immediately be applied to human patients with the disease
Investigational monoclonal antibody to treat Ebola is safe in adults
NIH clinical trial of remdesivir to treat COVID-19 begins
APEIRON’s respiratory drug product to start pilot clinical trial to treat coronavirus disease
CORAT Therapeutics obtained regulatory authorization for clinical phase Ib/II trial with the SARS-CoV-2 neutralizing human antibody COR-101 - COR-101 is a human antibody that blocks virus infection by binding to the spike protein

New muscle therapy gets fast-track boost - Berlin start-up could soon be helping children with previously incurable muscle diseases thanks to an accelerated approval process

SAS accelerates delivery of novel medicines using AI and analytics - Cooperation with AstraZeneca for more innovation in clinical research

Stockholm-based biotech start-up aims to develop the next generation of cell therapies - Neogap Therapeutics' PIOR technology identifies targets for personalised liver cancer immunotherapies

Millions in funding for the development of a novel drug against multi-resistant bacteria - Infex and Justus-Liebig-University Giessen awarded £1 million grant from PACE
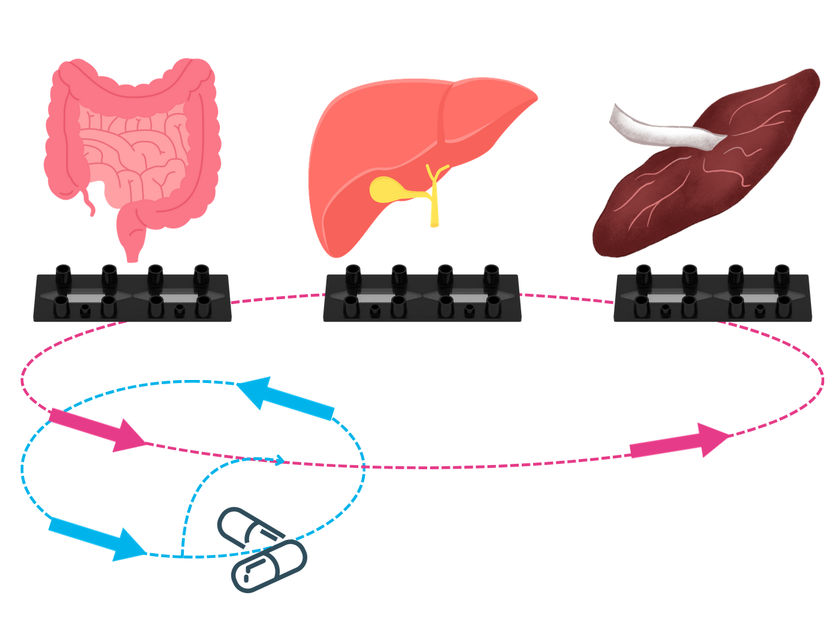
“Multi Organ Model” - Animal-free alternative for pharmacokinetics research - Dynamic42 and research partners present three-organ system to reduce animal testing