Biofrontera transfers clinical research to the U.S.
"We can be much more flexible in our strategy to expand our market presence in Europe thanks to the newly defined cost structure"
Advertisement
Biofrontera AG announces that Biofrontera AG and Biofrontera Inc. have agreed on an amendment to the existing license and supply agreement (LSA). This amendment provides that Biofrontera Inc. will take over the entire clinical development program, thus reducing the cost burden for Biofrontera AG.
The clinical study program, which includes the clinical studies for the market expansion of Ameluz® in the US, had already been defined in the original LSA between the two companies. Previously, Biofrontera AG had the responsibility and expenses to conduct this clinical trial program and received an agreed transfer price of 50% to 30%, depending on year sales level, of the U.S. sales price of Ameluz® as compensation.
The now agreed switch of responsibilities for the clinical program effective on or before June 1, 2024, will be temporarily offset by a decrease in revenues for Biofrontera AG. Over the next two years, Biofrontera AG will receive 25% of the U.S. sales price, with this share increasing again from 30% to 35% in the following years.
Through this agreement, Biofrontera Inc. is now able to manage its own clinical program, taking into account the needs of the U.S. market, while Biofrontera AG will not have to incur any further costs for clinical developments for the U.S. market. As a result, Biofrontera AG's available resources can now be focused on expanding its portfolio and growing the market in Europe and other countries.
"The strategic agreement allows both companies to focus more clearly on their core businesses. We can be much more flexible in our strategy to expand our market presence in Europe thanks to the newly defined cost structure. In addition, Biofrontera can continue to benefit significantly from the positive growth of the U.S. business. We are therefore very pleased about this new agreement with Biofrontera Inc., which now offers clear advantages for both companies," commented Pilar de la Huerta, Chief Financial Officer of Biofrontera AG.
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Stockholm-based biotech start-up aims to develop the next generation of cell therapies - Neogap Therapeutics' PIOR technology identifies targets for personalised liver cancer immunotherapies

Millions in funding for the development of a novel drug against multi-resistant bacteria - Infex and Justus-Liebig-University Giessen awarded £1 million grant from PACE
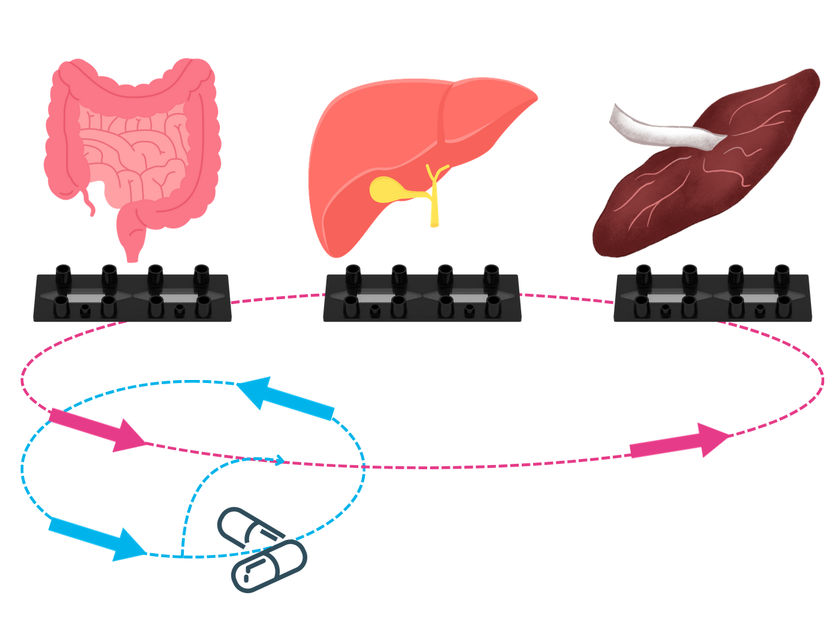
“Multi Organ Model” - Animal-free alternative for pharmacokinetics research - Dynamic42 and research partners present three-organ system to reduce animal testing

New methods for reliable evaluation of chemicals and pharmaceuticals - 18 partners from eight countries are working on the introduction of innovative methods for evaluating safety and efficacy without animal testing

New approaches in cancer research - Tumour-on-chip model supports preclinical drug development on pancreatic cancer

The EPO unveils the top 10 innovators of the first standalone edition of the Young Inventors Prize 2025 - Prize recognises innovators under 30 who are tackling Global Challenges through groundbreaking science and technology

NUCLIDIUM Closes EUR 84 Million Series B Financing - "NUCLIDIUM’s platform stands out in a rapidly evolving field and will change how radiotheranostic care is delivered"

Life Science Pitch Day: Where Visionaries Meet Investors - Start-ups presented their innovative approaches to solving urgent medical challenges

AmphiStar secures €12.5m EIC funding to bring its biosurfactants to market - Belgian start-up impresses with sustainable microbial biosurfactants, upcycled from a bio-based waste feedstock

Double Excellence: ibidi Recognized for Outstanding Business Success and Family Friendliness

BIOTECH AUSTRIA calls for better framework conditions for the biotechnology sector of the future - Proposals provide impetus for research, infrastructure and investments in Austria